Delocalized and hypothetically localized structures of these systems are thoroughly optimized and analyzed.
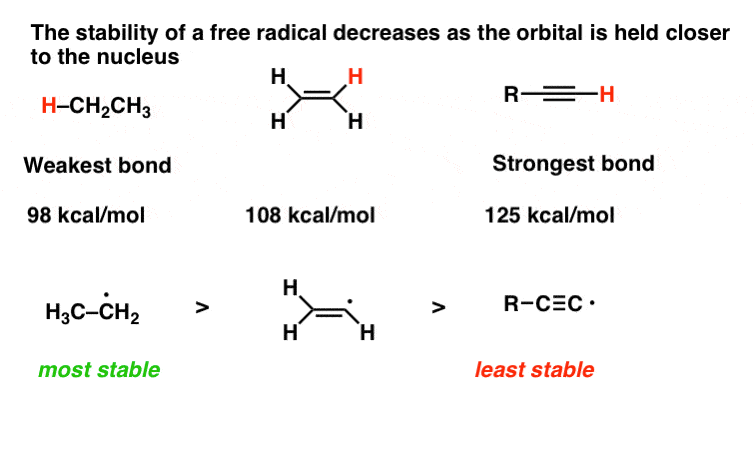
Difference between allyl and vinyl radical.
The delocalization energies defined as the energy difference between the delocalized structure and its hypothetically localized one for the three allyl systems are 55 7.
The terms allyl and vinyl are common in organic chemistry because we can use these terms to name compounds using.
An allyl group is a substituent with the structural formula h 2 c ch ch 2 r where r is the rest of the molecule.
As nouns the difference between allyl and aryl.
The allylic carbon atom is more reactive than normal.
The key difference between allyl chloride and vinyl chloride is that ally chloride contains its chlorine atom bonded to the carbon atom that is adjacent to the double bond whereas vinyl chloride contains its chlorine atom bonded to one of the two carbon atoms in the double bond.
Allyl and vinyl are the two different organic functional groups that hold differentiable characteristic and features.
Key difference allyl vs vinyl both allyl and vinyl groups have slightly similar structures with a small variation.
In context organic chemistry lang en terms the difference between allyl and aryl is that allyl is organic chemistry an organic radical ch 2 ch ch 2 existing especially in oils of garlic and mustard while aryl is organic chemistry any univalent organic radical derived from an aromatic hydrocarbon by removing a hydrogen atom.
Ab initio valence bond calculations are performed for the allyl cation radical and anion with 6 31g basis set.
Allyl groups have three carbon atoms and five hydrogen atoms.
Allyl indicates a functional group with structural formula h 2 c ch ch 2 r where r is the rest of the molecule it consists of methylene bridge ch 2 in between the vinyl group ch ch 2 and the rest of the molecule therefore allyl group contains sp 2 hybridized vinyl carbon atoms and sp 3 hybridized allyl carbon atom.
The molecular formula of the ally carbon group is rch2ch ch2 while that of the vinyl group is rch ch2.
The key difference between these two structural components is the number of carbon and hydrogen atoms.
The number of carbon and hydrogen in the ally group are three carbon atoms and five hydrogen atoms whereas that of the vinyl group is two carbon atoms and three hydrogen atoms.
The name is derived from the latin word for garlic allium sativum in 1844 theodor wertheim isolated an allyl derivative from garlic oil and named it schwefelallyl.
Both groups own a double bond between two carbon atoms where all the other atoms are bonded through single bonds.